Albert Guskov is a prominent structural biologist working on various membrane proteins (metal, vitamin, and amino acid transporters) and large molecular machineries (ribosomes and photosystems) using the combination of macromolecular crystallography, single particle cryo-electron microscopy and molecular dynamics simulations. He was trained in structural biology at the Institute of Protein Research of Russian Academy of Sciences (MSc, 2005) and in 2009 he received his PhD degree at Free University of Berlin under supervision of the renown crystallographer Prof Dr Wolfram Saenger. In 2010-2012 Albert stayed at the Nanyang Technological University in Singapore as a postdoc with Said Eshaghi, where he started working on membrane transporters and channels. In 2013, he moved to the Netherlands, where he built his career from a senior PostDoc to Associate Professor and currently he is the head of Biomolecular X-ray crystallography laboratory at the University of Groningen; from 2019 to 2022 he was also a visiting professor at the Moscow Institute of Physics and Technology, where he was the head of cryo-Electron Microscopy laboratory. Albert Guskov has published over 50 scientific publications (>3400 citations, h-index 25) and is regularly invited as a speaker to the national and international scientific meetings. Guskov’s lab at the University of Groningen combines state-of-the-art techniques of structural biology, namely macromolecular X-ray crystallography, single-particle cryo-electron microscopy and molecular dynamics simulations to tackle various important biological questions. The main research lines of the lab are as follows:
- Membrane transporters involved in metal homeostasis. We investigate the structural basis of (heavy) metal transport across biological membranes, such as Mg2+, Co2+, Ni2+, Zn2+, and Al3+. Most of these cations are toxic at high concentrations; therefore, tight regulation of their influx and efflux is essential for (micro-) organisms to survive. Cobalt, nickel and zinc are essential trace microelements, involved in numerous intracellular processes, such as nitrogen metabolism, carbon dioxide fixation, synthesis of enzymes and many others. The intracellular concentration of these essential divalent cations must be kept under tight control as lack of these metals will eventually lead to cell death and elevated concentrations are toxic. We have thoroughly characterized the family of CorA-related proteins and resolved their transport mechanism (Stetsenko, et al., 2021; Nemchinova et al., 2021; Stetsenko et al., 2020; Gati et al., 2017).
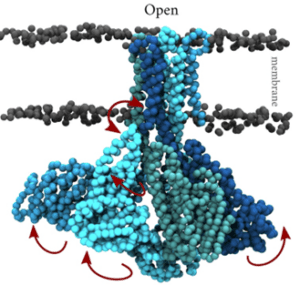
- Membrane transporters of Solute carrier family 1. This is a family of excitatory neurotransmitter transporters and neutral amino acid exchangers with a model protein from an archaeal homologue. Over the years, in collaboration with Slotboom’s lab, we have scrutinized the transport mechanism of this family and characterized numerous states both structurally and functionally (Stehantsev et al., 2021; Trinco et al., 2021; Arkhipova et al., 2020; Garaeva et al., 2019; Arkhipova et al., 2019; Garaeva et al., 2018; Arkhipova et al., 2017). Furthermore, in collaboration with Szymanski lab we have developed photoswitchable compounds for these transporters Arkhipova et al., 2021) which we are using for time-resolved studies.
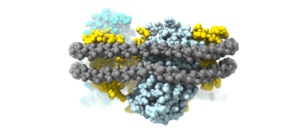
- Large cellular machineries – we are looking into yet unresolved questions in the enigmatic large complexes, such as photosystems and ribosomes. We have discovered a novel general dimerization mechanism in ribosomes (Franken et al., 2017), and recently we have resolved first structure of a ribosome from an infamous pathogen Candida albicans (Zgadzay et al., 2022), which can be used for the development of novel antifungal drugs.
- Last but not least we are studying several bacterial carbohydrate-processing enzymes involved in the synthesis of α-glucans from sucrose or from starch-like substrates (Gangoiti et al., 2017). Understanding how the structures of these enzymes determine their reaction specificity (Bai et al., 2017; Pijning et al., 2021) supports and advances the development of their application in the food industry for the production of low-glycemic ingredients (e.g., dietary fibers).

